

Iso 17025 2017 iso#
Less time administering your ISO 17025 quality system means you'll have more time to grow your business. Our goal is to make gaining and maintaining ISO 17025 accreditation as simple and efficient as possible. Yet, it is a necessary part of doing business as a test lab. The transition period for the conversion to the new ISO/IEC 17025:2017 standard is three years from the date of publication of the international standard, giving accredited laboratories until 30 November 2020 to apply to have their accreditations based on the old standard adapted.ĭAkkS recommends converting to the new standard within the framework of regular surveillance assessments or applying for and implementing re-accreditation.Īfter completing its preparations and the training of its auditors, the accreditation body will be able start carrying out assessments on the basis of the new ISO/IEC 17025:2017 standard from 1 July 2018.ĭAkkS has published further information in a separate guide to conversion. ISO/IEC 17025:2017 is applicable to all organizations performing laboratory activities, regardless of the number of personnel. Conforming to ISO/IEC 17025:2017 can be time consuming and tedious. Compliance with the standard makes it possible to determine laboratory results in a technically competent manner and ensure their international acceptance. The standard sets out the general requirements with respect to the competence of testing and calibration laboratories, and also forms the basis for accreditation in this area.

Please note that throughout this article the term the standard refers. The document is expected to proceed to publication, planned for end November/December 2017. G The deliverable of this meeting was FDIS version of the new ISO/IEC 17025 versionthe. We have broken down the ISO/IEC 17025:2017 standard in hopes to make it easier. ISO/CASCO WG 44 meeting was held on July 10-12, 2017 in ISO Central Secretariat, eneva. The German version of the standard is due to appear in spring. Ce document a pour objectif d’expliciter les exigences de la norme NF EN ISO/IEC 17025 version 2017, prise en compte pour laccréditation des laboratoires. General Requirements for the Competence of Testing and Calibration Laboratories. The new version of the globally applicable standard for the laboratory sector was drawn up by the competent committee for conformity assessment (CASCO) at the international standardisation body ISO and published in English at the end of November.
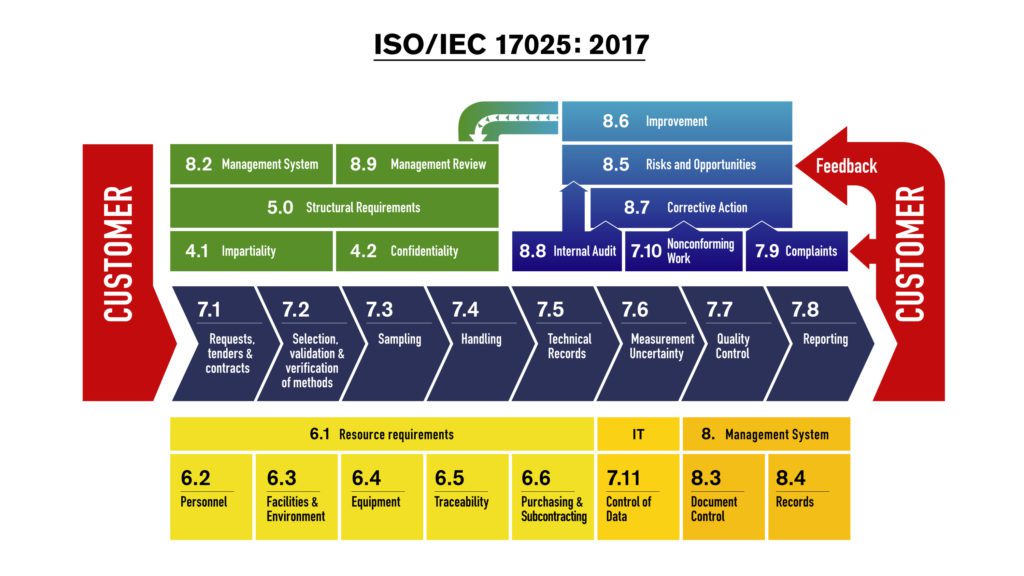
German Accreditation Body (DAkkS) is currently preparing the process of conversion to the ISO/IEC 17025:2017 standard.

Key topics include testing and calibrating using standard, non-standard and laboratory-developed methods under controlled conditions. ISO/IEC 17025:2017 Compliance Introduction for Technicians. ISO/IEC 17025 checklist is considered a very good tool for auditors to make an audit questionnaire to verify the effectiveness of the implemented laboratory management system. This standard applies to everything from test equipment to data sampling and sets out valid specifications to improve an organisation's quality management systems and as a result, their quality control. ISO/IEC 17025 checklist covers audit questions based on ISO/IEC 17025:2017 requirements for each department of the testing and calibration laboratories as given below. BS EN ISO/IEC 17025:2017 is a set of requirements that calibration laboratories and testing organisations have to meet to prove that they operate a quality system, are technically competent and can generate technically valid and repeatable results.


 0 kommentar(er)
0 kommentar(er)
